Denmark and Norway has suspended AstraZeneca Covid-19 vaccine, similar to what Malawi got, over fears that some patients were having blood clots after the jab but European authorities say there is no evidence linking the clots to the vaccine.
News Site Aljazeera reported that Denmark will not use AstraZeneca’s COVID-19 vaccine for two weeks after reports that some recipients had developed serious blood clots.
A 60-year-old woman who was given the vaccine also developed clots and died.
“It is currently not possible to conclude whether there is a link. We are acting early, it needs to be thoroughly investigated,” Health Minister Magnus Heunicke said on Twitter.
According to A Jazeera, following Denmark’s move, Norway announced later on Thursday that it was also halting the use of the AstraZeneca vaccine.
“This is a cautionary decision,” Geir Bukholm, director of infection prevention and control at the Norwegian Institute of Public Health (FHI), told a news conference.
The European Union’s drug regulator, the European Medicines Agency (EMA), said on Wednesday there was no evidence so far linking AstraZeneca to two cases in Austria where there was a death from coagulation disorders and an illness from a pulmonary embolism.
Meanwhile, AstraZeneca has defended its vaccine saying studies have shown that the vaccine was generally well tolerated.
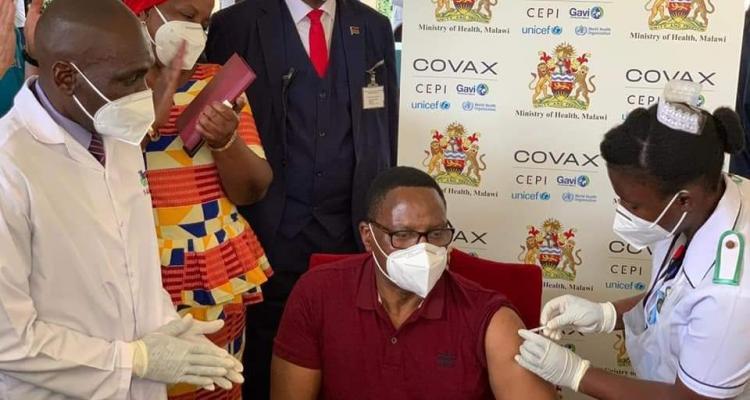
The reports come as Malawi has today launched its Vaccination programme. The country received 360,000 doses of the AstraZeneca vaccine last week and President Lazarus Chakwera got vaccinated today.














